Unique Tips About How To Write Synthesis Reactions

A synthesis reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product.
How to write synthesis reactions. A synthesis reaction is a chemical reaction in which two or more reactants combine to form a single product. Predict the products (100 examples) chemistnate. 0:00 / 4:16.
Follow an example multistep synthesis. Describes the basics of synthesis reactions, how to identify them, predict the product and balance the chemical equation. A combination reaction, also known as a synthesis reaction, is a reaction in which two or more substances combine to form a single new substance.
General form of synthesis reactions. Synthesis reactions (also called combination reactions) are the simplest type of chemical reaction. In this chapter, we’ll learn about designing a synthesis that involves more than the one or two reactions we’ve seen before.
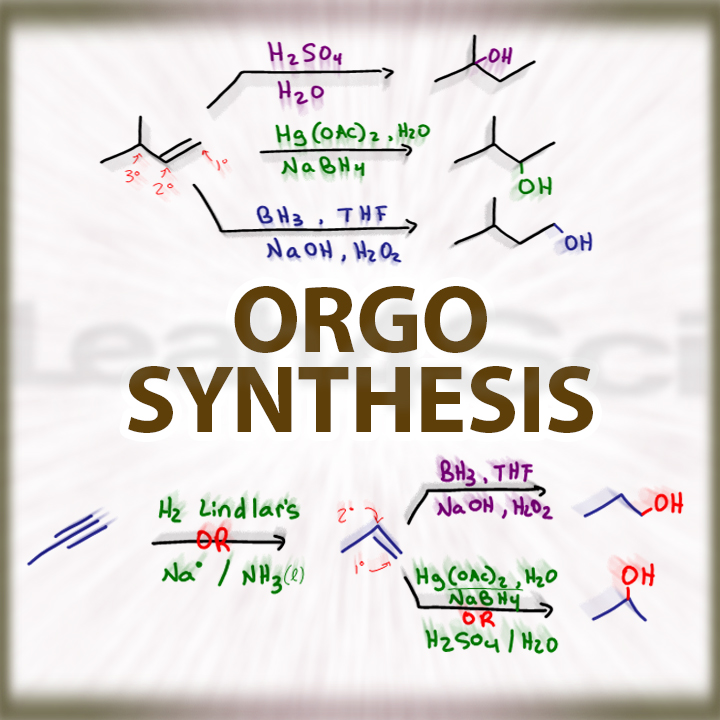
A synthesis reaction or direct combination reaction reacts two or more simple elements or compounds to form a more complex product. This organic chemistry video tutorial focuses on multistep synthesis reactions and retrosynthesis problems. 1) direct union of two elements will.
A combination reaction is a reaction in which two or more substances combine to form a single new substance. This can be accomplished either as an ionic or molecular coupling in the. A synthesis reaction is is the combining of two single elements into a compound.
6.4k views 1 year ago. A + b → ab. 19k views 3 years ago grade 11 chemistry.
This article defines the role that continuous flow chemistry can have in new reaction discovery, thereby creating molecular assembly opportunities beyond our. Categories apply, just in reverse. It contains plenty of tips, techniques, examples.
Chemical compounds are made up of atoms of different elements, joined together by chemical bonds. What do you do when a transformation can’t be done in one step? Multiple stepwise products with the proper reaction's reagents, solvent, and conditions.
Here are some examples of synthesis. The reactants may be elements or compounds, while the product is always a compound. Since synthesis reactions are the reverse of decomposition, you might ask if the decomp.
The product will contain both elements. Writing out a synthesis. First, we need to see.






.PNG)






:max_bytes(150000):strip_icc()/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)
.png)